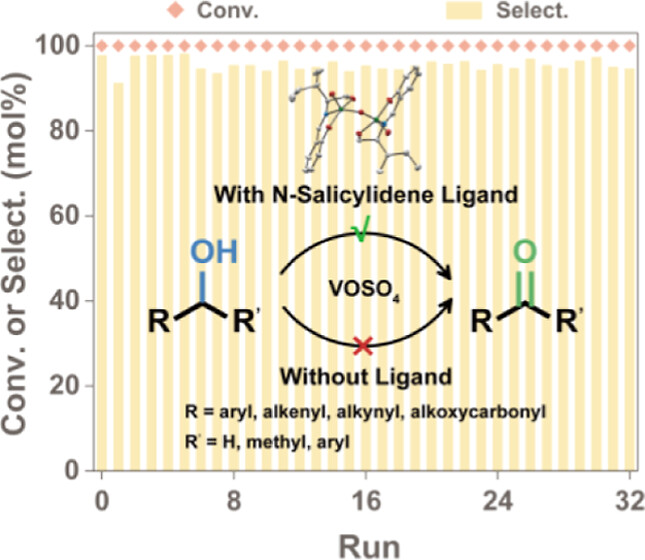
In this study, our focus lies in investigating how the microenvironment surrounding the vanadium center influences the selective aerobic oxidation of alcohols via a tailored ligand design. Under optimized reaction conditions, oxovanadium complex 1a with N-salicylidene-amino alcohol ligands effectively converted various hydroxyl compounds, including primary benzylic, allylic, propargylic, heteroaryl-containing alcohols, lactate, and secondary aromatic alcohols, into their corresponding aldehydes or ketones in high yields without any additives. Complex 1a displayed remarkable reaction stability and featured a binuclear structure containing VV species confirmed by vanadium nuclear magnetic resonance and single-crystal X-ray diffraction analysis. Activating the α-C−H bond is the rate-determining step verified by the kinetic isotopic effect study. The introduction of electron-donating groups on the ligand’s aromatic ring could increase its catalytic activity. Mechanistic studies indicate that the oxovanadium complex undergoes a cycle from VV species to VIV species, validated by electron paramagnetic resonance. These phenomena have implications for the development of efficient metallic complexes for aerobic oxidation processes.